Abstract
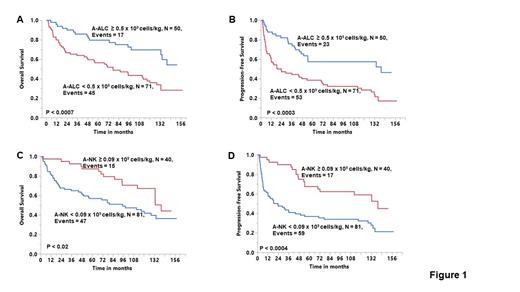
Our group published a double blind, randomized phase III study (Porrata et al, BBMT 2016; 22: 1017-1023) showing that the infusion of autograft absolute lymphocyte count (A-ALC) ≥ 0.5 x 10 9 cells/kg is a survival prognostic factor for lymphoma patients undergoing autologous peripheral blood hematopoietic stem cell transplantation (APBHSCT). However, a limitation of the study was the short-term follow-up duration of two years post-APBHSCT. To assess if the A-ALC still provide survival benefit we looked at the 121 lymphoma patients enrolled in the phase III from December 10, 2007 until October 12, 2010 survival outcomes with a longer follow-up duration of up to 13 years post-APBHSCT. Since the accrual for the study, 52 patients had died of recurrence lymphoma, 4 patients of therapy-related acute myelogenous leukemia, 3 patients of unknown causes, 1 patient of acute respiratory distress syndrome, 1 patient of anaplastic astrocytoma, and 1 patient of septic shock. The current median follow-up is 86 months (range: 2.1-158.1 months) for the entire cohort and 127.8 months (range: 5.9-158.1 months) for patients still alive. Patients infused with an A-ALC ≥ 0.5 x 10 9 cells/kg continue to experience superior overall survival (OS) (HR = 0.392, 95%CI 0.224-0.687, P < 0.001) and progression-free survival (PFS) (HR = 0.413, 95%CI 0.253-0.677, P < 0.0004). The 10-year OS rates for the A-ALC ≥ 0.5 x10 9 cells/kg patients was 70% (95% confidence interval [CI], 55%-81%) and for the A-ALC < 0.5 x10 9 cells/kg patients was 36% (95% CI, 25%-49%) (Figure 1A). The 10-year PFS rates for the A-ALC ≥ 0.5 x10 9 cells/kg group was 57% (95% CI, 43%-70%) and for the A-ALC < 0.5 x10 9 cells/kg group was 28% (95% CI, 19%-40%) (Figure 1B). Autograft lymphocyte subset analysis including autograft-CD3 (A-CD3), autograft CD4 (A-CD4), autograft CD8 (A-CD8), and autograft natural killer cells (A-NK), showed that the infusion of A-NK was a predictor for OS (HR = 0.518, 95%CI 0.292-0.919, P < 0.02) and PFS (HR = 0.393, 95%CI 0.230-0.670, P < 0.0006). The 10-year OS rates for the A-NK ≥ 0.09 x10 9 cells/kg patients was 67% (95% confidence interval [CI], 50%-80%) and for the A-NK < 0.09 x10 9 cells/kg patients was 42% (95% CI, 31%-54%) (Figure 1C). The 10-year PFS rates for the A-NK ≥ 0.09 x10 9 cells/kg group was 59% (95% CI, 43%-73%) and for the A-NK < 0.09 x10 9 cells/kg group was 32% (95% CI, 23%-44%) (Figure 1D). Both the A-ALC and A-NK were independent predictor for OS [ A-ALC: HR = 0.367, 95%CI 0.195-0.690, P < 0.001) and A-NK: HR = 0.411, 95%CI 0.275-0.892, P < 0.01] and for PFS [ A-ALC: HR = 0.477, 95%CI 0.271-0.840, P < 0.01) and A-NK: HR = 0.490, 95%CI 0.266-0.903, P < 0.02]. With a longer follow-up of 13 years, this study shows that the infusion of A-ALC continues to provide better clinical outcomes to lymphoma patients undergoing APBHSCT. These study findings support our standard practice changed of not only collecting CD34 stem cell for hematologic engraftment, but also A-ALC for lymphoma patients undergoing APBHSCT to improve clinical outcomes. Further, autograft immune effector studies are warranted such as A-NK to develop a more immunocompetent targeted autologous graft verus tumor effect.
Ansell: Bristol Myers Squibb, ADC Therapeutics, Seattle Genetics, Regeneron, Affimed, AI Therapeutics, Pfizer, Trillium and Takeda: Research Funding. Paludo: Karyopharm: Research Funding.